

The magnesium we absorb is never pure magnesium. Magnesium ions (Mg2+) are highly reactive and unstable on their own. They are positively charged ions and like to form compounds with other substances. When they bind with negatively charged molecules (anions) they form salts. When they bind specifically with amino acids, we call them amino acid complexes.
How we absorb salts and complexes are slightly different. We’ll show you how that works.
Before magnesium from salts can be absorbed, they must first be dissolved back into ionic forms. The strong acids of the stomach break apart the bonds that hold magnesium to the other molecules. In this soluble, fluid state, the free magnesium ions travel down to the duodenum where they are absorbed through small mineral ion channels found along your intestinal wall.
Pretty straightforward so far, but magnesium ions encounter a few challenges. The ion channels are small and can only transport magnesium ions when they are unattached to other molecules.
That’s easy to ensure in the gastric acid of your stomach – acidic environments are good at breaking the bonds between magnesium and other molecules. But the further away you travel from the stomach, the less acidic the environment becomes. The less acidic the environment, the harder it is for magnesium ions to remain soluble.
What happens when the pH levels rise? Magnesium ions start to bind with other nearby substances, usually forming insoluble compounds. These insoluble compounds precipitate out of the liquid and pass through your body as waste!
Think about the calcium deposits that sometimes clog your showerhead. There is always a certain level of calcium ions that are in the water. At cool temperatures, this isn’t a problem. Calcium ions will remain dissolved in water.
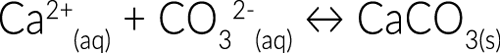
But we like hot showers. As the water heats up, calcium begins to bind with carbonate ions also dissolved in the water. This forms insoluble calcium carbonate which precipitates out of the water and forms solid deposits on the inside of your showerhead.
There’s another challenge. Even if a magnesium ion remains dissolved, its positive electrical charge will tend to attract water molecules. A shell of water (a hydration shell) will start to form around magnesium, making it rather large. The hydrated radius is about 400 times larger than the radius of a bare magnesium atom.
A sizeable challenge for absorption but not insurmountable. The proteins that make up the ion channels are capable of removing enough water for the magnesium ion to pass through to the far side.
 The caveat? These proteins need a certain acidic pH to function properly.
The caveat? These proteins need a certain acidic pH to function properly.
Magnesium salts are a good source of magnesium provided you can keep your pH low enough.
Magnesium amino acid complexes (or chelates) behave differently from magnesium salts. The strong and stable bonds between magnesium and the amino acids keep the whole molecule intact in acidic environments.
When complexes travel through the intestinal tract, they bypass the ion channels. Instead, they use other transport sites called dipeptide channels. Here, the amino acid and magnesium are carried across the intestinal membrane together.
There are a few key advantages to this. For one, there are far more dipeptide channels than there are ion channels in the gut. Magnesium complexes do not compete for the same ion channels used by other minerals.
Another advantage is that the stable bonds protect magnesium from unfavourable chemical reactions that might lead to the creation of unabsorbable precipitates.
Mineral amino acid complexes are actually quite common in nature and a natural way we get magnesium from our diet. Some of this magnesium is already bound to amino acids, but even when we consume magnesium from salts, amino acids can help!
Peptides and amino acids can bind to magnesium ions and form complexes right in your body.
It’s all about osmosis. When there are more mineral ions unabsorbed in the intestinal tract than there are mineral ions in the surrounding intestinal cell wall, osmosis tries to equalize the concentration of ions.
This means water flows out of the mucosa cells into the intestine. Too much water in the intestinal tract could mean loose stools, bloating and diarrhea. That’s why having too much unabsorbed magnesium in your gut can have this laxative effect! Yuck!
Studying the bioavailability of magnesium is complicated. Unlike pharmaceutical drugs that can be detected chemically in the body, magnesium is found everywhere in the body in high concentrations. Direct measurement is also complicated. Our blood contains less than 1% of our total magnesium levels, is tightly regulated, and changes by up to 6% depending on the time of day. Even if we test for magnesium in blood serum, we’re also unsure how magnesium levels in the blood relate to magnesium levels in bones or in soft tissues.
All these things make it difficult to clinically determine how much magnesium is retained by the body through measuring blood or urine.
However, we can reliably investigate how well different magnesium types are absorbed through the intestinal walls.
Many pharmaceutical scientists study the absorption of drugs by cultivating intestinal cells into a thin membrane layer that has all the features of a real intestinal wall, including various nutrient transport channels and a brush border. This layer of cells is placed into special plates called transwells. From these, scientists can measure how much of an agent permeates through this semipermeable layer to the other side.
Transwells have two compartments separated by a thin membrane of cultivated intestinal cells. Researchers place different magnesium solutions into the inner compartment and measure how much magnesium passes through the membrane into the outer compartment.
Scientists studying magnesium absorption have also used this method to predict how well different kinds of magnesium can pass through our intestinal lining. First, different kinds of magnesium are added to a solution that mimics the pH of the digestive tract. They are placed into the upper compartment of these plates. Some of the magnesium will transport through this membrane layer into the bottom compartment. The bottom compartments are then measured for magnesium levels using a mass spectrometer.
These are fast and repeatable experiments that help us build a model for understanding how magnesium is absorbed in the body.
So what are the results? A 2016 study using the above methodology resulted in the following.
The chelated magnesium bis-glycinate and buffered magnesium bis-glycinate (the same materials we use) were much better absorbed than other forms like magnesium citrate and magnesium oxide on its own.
Learn about how magnesium works, why it’s important, and how it can help you.

Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information.
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via analytics, ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
These cookies collect information about how visitors use a website, for instance which pages visitors go to most often, and if they get error messages from web pages. These cookies don’t collect information that identifies a visitor.
The “Other” category cookies help us provide our visitors proper functionality of the website such as online quizzes or embedded videos.
Analytics cookies help us understand how our visitors interact with the website. It helps us understand the number of visitors, where the visitors are coming from, and the pages they navigate. The cookies collect this data and are reported anonymously.
Advertisement cookies help us provide our visitors with relevant ads and marketing campaigns.